Anesthesia and Physics Review
Respiratory
Gas Laws
Ideal Gas Law
Pressure, Volume, number of moles, universal gas constant, temperature
\[PV = NrT\]Boyle’s Law
This law describes an inverse relationship. An example is the diaphragm contracting or shortening and increasing tidal volume.
\[P_1V_1 = P_2V_2\]Charles’ Law
\[{V_1 \over T_1} = {V_2 \over T_2}\]Gay Lussac’s Law
\[{P_1 \over T_1} = {P_2 \over T_2}\]Henry’s Law
At a constant temperature the amount of gas that dissolves in the solution is directly proportional to the partial pressure of the gas over the solution…aka higher the gas pressure the more dissolves (overpressurizing the vaporizer). But if temp increases, then the solubility goes down and vice versa…so dec temp inc solubility and therefore prolongs emergence since the gasses are not just staying in the lungs or in circulation. Henry’s constant is more of a coefficient since its value is temperature dependent.
\[Partial Pressure = Contstant * Concentration\]Dalton’s Law
\[P_total = P_1 + P_2 + P_3...\]Alveolar Gas Equation
$ P_a $ is Atmospheric Pressure and $ P_w $ Pressure of water vapor
\[PAO_2 = FiO_2(P_a - P_w) - {PaCO_2 \over RQ}\]Dead Space
Bohr Equation
\[{V_D \over V_T} = {P_aCO_2 - P_ECO_2 \over PaCO_2}\]Spontaneous Ventilation
\[{150 \over 450} = {2ml/kg}\]Controlled Ventilation
Increased $V_D$ due to decreased BP
Respiratory Quotient Derivation
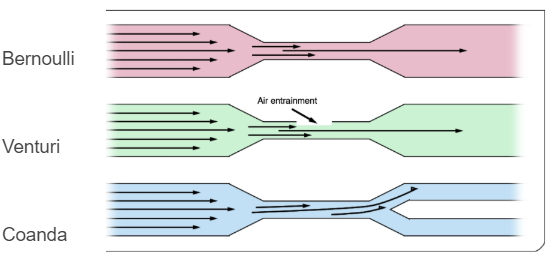
\[{R} = {P_ECO_2(1-FiO_2) \over P_iO_2 - P_EO_2 - (P_ECO_2 * FiO_2)}\] \[{R} = {CO_2 Production \over O_2 Consumption}\] \[{R}= {200 \over 250} ={0.8}\]Fluid Dynamics Review
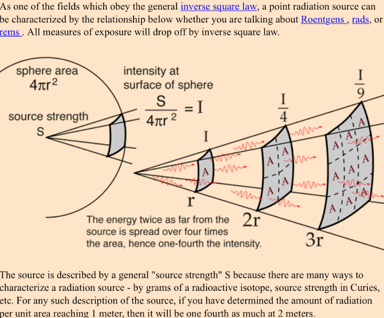
Inverse Sqare Law and Radiation Safety
Time Constant and the Circuit
Volume of the circuit is a known constant and the total fresh gas flow FGF is Q. Three time time constants τ are required for 95% change in concentration. So as the FGF is decreased the time to equillibration is increased.
\[{V \over Q} = \tau\]